VincentG wrote: ↑Mon Apr 15, 2024 3:47 am
...
I tend to believe that in a piston engine, heat is largely not converted to work and that it does infact flow through the engine, if work is being done.
.....
After what must have been several years of reading all kinds of conflicting views and theories spanning centuries, I didn't know what to think, and never cared one way or the other what theory or who's theory might be right.
What was the experimental evidence?
Mostly everything I read said there was no experimental evidence relating to the Carnot theory because it would be impossible to build an actual Carnot engine.
Well, to me, if true, that's a red flag smacking of actual "pseudoscience" based on Popper's principle of falsifiability.
But, we have an actual mathematical equation that, based on the Carnot principle, or some Kelvin-esque derivation therefrom, purports to inform us of exactly how much heat we should be able to find being "rejected" from a "perfect" Carnot engine.
A real engine, being LESS efficient than a theoretical Carnot engine should be found to "reject" MORE "waste heat" than a Carnot engine.
So, measuring the heat being "rejected" from a Stirling engine, we could determine which theory was correct.
At no time should we find LESS heat being rejected from a real engine than predicted by the Carnot efficiency formula for a Carnot engine, since a Carnot engine is supposed to be more efficient, it would always produce less "waste heat" than any real engine. If the Carnot equation and general theory or "LAW" is correct a real engine will always reject MORE waste heat than the formula predicts for a Carnot engine.
Numerous sources for how to calculate the expected heat "rejected" to the "cold reservoir" for a Carnot engine can be found in abundance. There seems to be no controversy about that
In no case whatsoever, if the Carnot limit theory and formula are true, should we find NO HEAT WHATSOEVER being rejected to the "cold reservoir" or "sink" for ANY REAL engine.
Experimentally, however, that appears to be the case.
In all my experiments scarcely any heat at all can be found leaving the cold side of my model Stirling engines and in some instances NO heat could be detected. In a few instances the cold side appeared, by my temperature measuring instruments, to actually get a degree or two COLDER than the ambient "cold reservoir".
This is supported by this alleged graph showing actual temperature readings from a real engine
:
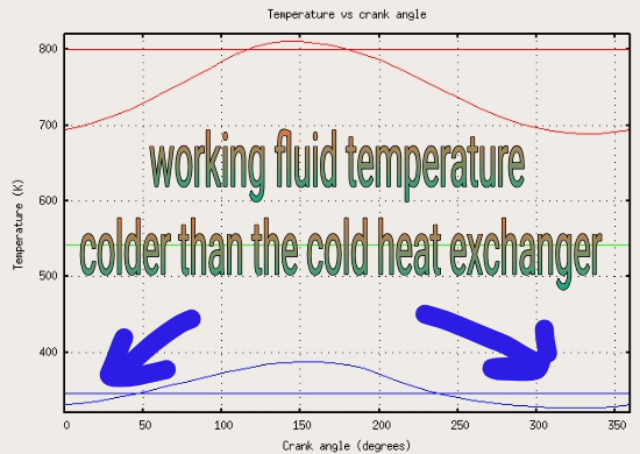
- Carnot-efficiency-falsification.jpg (186.09 KiB) Viewed 221 times
So, it appears from such temperature readings, that such experimental results (cooling of the working fluid BELOW the temperature of the sink) is not impossible.
There is abundant additional evidence from various fields that further support these conclusions.
Gases compressed and then expanded in an engine so to convert heat into work, in this way removing heat is the basis of many industrial processes: air-cycle (cryogenic) refrigeration, gas liquefaction etc.
So, my disinterested, unbiased conclusion is, the Carnot formula is invalid
Joule's results were correct.
Tesla was correct.
I have no steak in the outcome one way or the other. Just trying to resolve a debate that has gone unresolved for two centuries already.
I agree, and have stated many many times that my experiments are far from perfect and cannot be considered conclusive. The results should be checked and rechecked and the experiments should be conducted by other researchers and scientists with better, more accurate instrumentation.
I posted the results, or apparent results to the various science forums, inviting criticism and expert opinion, in hopes that some might replicate, or at least attempt to replicate such experiments.
Instead I'm attacked and perma-banned, for seeking some semblance of "peer review". Though not considering myself in any way a "peer" to the university professors or whomever. I just humbly requested some feedback from some "experts" in the field of thermodynamics and such.
I did not even know if the results were necessarily unusual. Should ice melt more slowly when running a Stirling engine, because SOME of the heat is converted to work, so there is LESS heat available to melt the ice?
Seems like a sensible experiment that could yield important data.
Is the heat converted? Does it "disappear" inside the engine or pass through to the ice?
A very simple, logical, common sense experiments ANYONE can perform at their own kitchen table.
Well, my result was that the ice melted more slowly. The ice lasted longer when running a Stirling engine. It appears that LESS heat is passing through. At least SOME heat is being "consumed" or "disappears".
Ahhh!!!!!!
perpetual motion!!!!!!
A Tesla nut!!! Lock the thread, ban him from the forum!!!